Internal standards play a pivotal role in ensuring accuracy and consistency in drug development. They serve as reference points, making analytical procedures reliable and comparable across different laboratories and studies. Within the drug development process, especially in bioanalysis, internal standards are indispensable. They help correct for variability that might arise during sample preparation, instrument fluctuations, and environmental changes. Without these standards, the results could be inconsistent, leading to potential drug efficacy and safety issues. Understanding how and why internal standards function is vital for any pharmaceutical scientist or analyst involved in drug development.
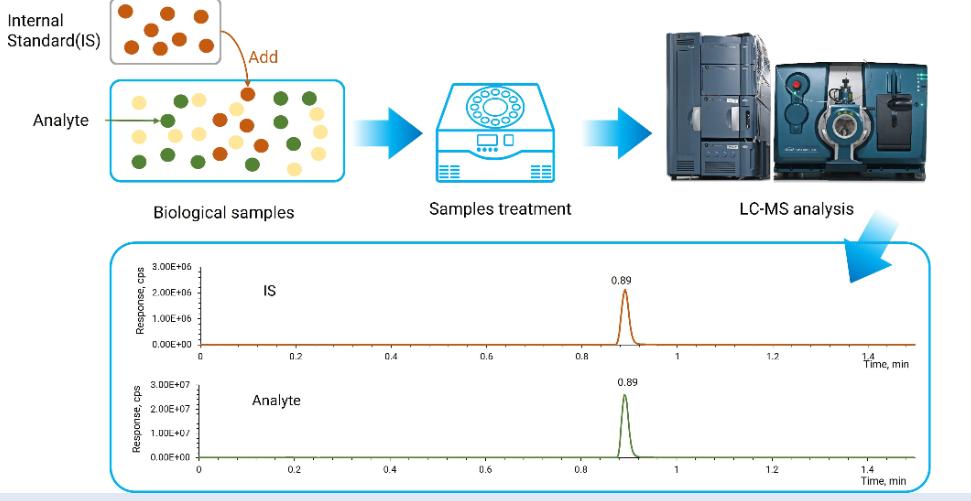
The Role of Internal Standards in LC-MS Bioanalysis
Definition and Purpose
An internal standard is a compound closely related to the analyte of interest used as a reference point during the analysis. Its primary purpose is to correct for potential errors and variability during the bioanalytical process. By comparing the analyte signal to the internal standard, this method provides a more accurate and reliable quantitative measurement. Thus, internal standards are essential for achieving precision in Liquid Chromatography-Mass Spectrometry (LC-MS).
How Internal Standards Correct Variability
Internal standards address variability by standardizing measurements taken across different samples. By spiking samples with a known quantity of the internal standard, analysts can compensate for variations in analytical conditions and sample preparation. This process ensures that the measured analyte’s response is solely due to its concentration, not inconsistencies in the procedure. Ultimately, this allows for more reliable data interpretation and quality control in drug development.
Choosing the Right Internal Standard
Stable Isotope-Labeled vs Structural Analogues
When selecting an internal standard, stable isotope-labeled compounds and structural analogues are two common choices. Stable isotope-labeled internal standards are identical to the analyte but include isotopes that distinguish them during analysis. This makes them ideal for eliminating matrix effects and enhancing accuracy. Alternatively, structural analogues are compounds closely related to the analyte’s structure but differ slightly. While generally less precise than isotopic labels, they are often more cost-effective and practical for certain applications.
Key Selection Criteria (Purity, Similarity, Stability)
When choosing an internal standard, purity, chemical similarity to the analyte, and stability are critical factors. High purity prevents interference in the analysis, ensuring results are solely due to the analyte. Chemical similarity promotes consistent behavior under experimental conditions. Stability is crucial, meaning the standard won’t degrade or react during the process, which would compromise the data. Understanding these criteria empowers researchers to make informed decisions in selecting the best internal standard.
Timing and Concentration: Crucial Factors
When to Add the Internal Standard
The timing of internal standard addition is crucial to its effectiveness. For best results, it is typically added early in the sample preparation process. This timing allows the standard to undergo the same processing steps as the analyte, correcting any variations across the entire procedure. By adding it early, analysts can monitor and adjust for any potential losses or alterations that may occur, ensuring analytical fidelity.
Determining the Ideal Concentration
Choosing the right concentration of an internal standard is vital. An ideal concentration is similar to that of the analyte, enabling accurate correction of variability. A concentration too low may not be detectable, resulting in unreliable data, while concentrations too high can cause saturation or matrix effects. A balance must be struck to ensure that the standard serves its purpose effectively, providing reliable data output and maintaining analytical consistency.
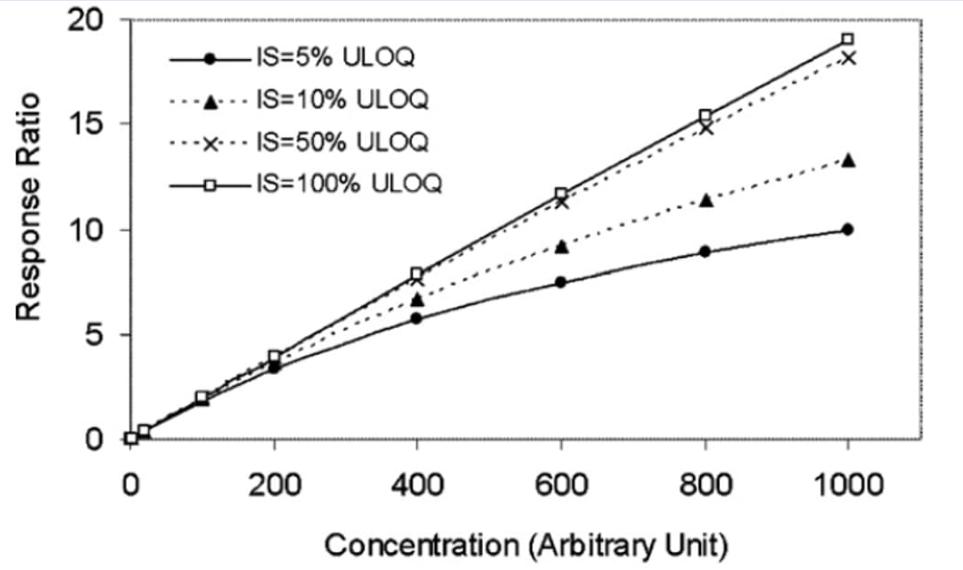
Evaluating Internal Standard Responses
Monitoring IS Response Variability
Regular monitoring of internal standard response variability is essential for ensuring analytical quality. Analysts track the internal standard’s response to identify any drift or inconsistencies over time. Significant deviations could indicate potential issues with the analysis process or sample integrity, prompting necessary investigations and adjustments. Continuous assessment ensures that results remain accurate and reliable throughout the study.
IS as Quality Control—“Friend” or “Foe” Cases
Internal standards can act as both quality control tools and points of failure, depending on their use. When correctly implemented, they serve as “friends,” enhancing data reliability and accuracy. In contrast, poor selection, incorrect implementation, or lack of monitoring can render them “foes,” misleading results and compromising data integrity. Consistent quality assessments and knowledgeable application ensure internal standards enhance, rather than hinder, analytical reliability.
Regulatory and Practical Impact in Drug Development
Internal standards significantly impact the regulatory and practical aspects of drug development. Regulatory bodies, such as the FDA, require precise and consistent analytical methods, where internal standards play a crucial role. They ensure compliance with regulations by providing reliable data used to support drug safety and efficacy claims. Practically, they simplify complex analyses, increase reproducibility, and reduce potential costs associated with inaccurate data. Their effective use is vital for meeting stringent regulatory standards and achieving successful drug development outcomes.
Conclusion
In drug development, internal standards are fundamental to achieving accurate and reproducible analytical results. From correcting variability to ensuring regulatory compliance, they enhance the reliability of data and the overall quality of the drug development process. Proper selection, timing, and concentration are essential for maximizing their benefits. Through diligent application and monitoring, internal standards remain indispensable tools in the pursuit of effective and safe pharmaceuticals.





Leave a Reply